Defenses against SARS-CoV-2 variants
Our key defense against the COVID-19 pandemic is neutralizing antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus elicited by natural infection or vaccination. Recent emerging viral variants have raised concern because of their potential to escape antibody neutralization. Wang et al. identified four antibodies from early-outbreak convalescent donors that are potent against 23 variants, including variants of concern, and characterized their binding to the spike protein of SARS-CoV-2. Yuan et al. examined the impact of emerging mutations in the receptor-binding domain of the spike protein on binding to the host receptor ACE2 and to a range of antibodies. These studies may be helpful for developing more broadly effective vaccines and therapeutic antibodies.
Science, abh1766, this issue p. eabh1766, abh1139, this issue p. 818
Structured Abstract
INTRODUCTION
Worldwide appearance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) with increased transmissibility and resistance to therapeutic antibodies necessitates the discovery of broadly reactive antibodies. We isolated receptor binding domain (RBD) targeting antibodies that potently neutralize 23 variants, including the B.1.1.7, B.1.351, P.1, B.1.429, B.1.526, and B.1.617 VOCs. Structural and functional studies revealed the molecular basis for antibody binding and showed that antibody combinations reduce the generation of escape mutants, suggesting a potential means to mitigate development of therapeutic resistance.
RATIONALE
Investigation of antibody responses from convalescent subjects infected with the Washington-1 (WA-1) strain for reactivity against WA-1 and VOCs can inform improvements to vaccine design and therapeutics.
RESULTS
Blood from 22 convalescent subjects who recovered from SARS-CoV-2 WA-1 infection was screened for neutralizing and binding activity, and four subjects with high reactivity against the WA-1 variant were selected for antibody isolation. SARS-CoV-2 spike (S)–reactive antibodies were identified through B cell sorting with S protein–based probes. WA-1 live-virus neutralization assays identified four RBD-targeting antibodies with high potency [half-maximal inhibitory concentration (IC50) 2.1 to 4.8 ng/ml], two of which were derived from the same IGHV1-58 germline but from different donors. Antigen-binding fragments (Fabs) of these antibodies exhibited nanomolar affinity to S (2.3 to 7.3 nM). Competition assays and electron microscopy indicated that two of the most potent antibodies blocked angiotensin-converting enzyme 2 (ACE2) and bound open conformation RBD, whereas the other two bound both up and down conformations of RBD and blocked ACE2 binding. Binding and lentivirus neutralization assays against 13 circulating VOCs or variants of interest—including B.1.1.7, B.1.351, B.1.427, B.1.429, B.1.526, P.1, P.2, B.1.617.1, and B.1.617.2—indicated that these antibodies were highly potent against VOCs despite being isolated from subjects infected with early ancestral SARS-CoV-2 viruses. Cryo-EM studies of the two most potent antibodies in complex with S revealed that these antibodies target a site of vulnerability on RBD but have minimal contacts with mutational hotspots, defining the structural basis for their high effectiveness against the emerging VOCs and further delineating an IGHV1-58 antibody supersite. To investigate potential mechanisms of escape, we applied antibody selection pressure to replication-competent vesicular stomatitis virus (rcVSV) expressing the WA-1 SARS-CoV-2 S (rcVSV-SARS2) and identified S mutations that conferred in vitro resistance. We evaluated these antibodies individually or in combinations for their capacity to prevent rcVSV-SARS2 escape and discovered that antibody combinations with complementary modes of recognition to the RBD lowered the risk of resistance.
CONCLUSION
Our study demonstrates that convalescent subjects previously infected with ancestral variant SARS-CoV-2 produce antibodies that cross-neutralize emerging VOCs with high potency. Structural and functional analyses reveal that antibody breadth is mediated by targeting a site of vulnerability at the RBD tip offset from major mutational hotspots in VOCs. Selective boosting of immune responses targeting specific RBD epitopes, such as the sites defined by these antibodies, may induce breadth against current and future VOCs.
Antibodies isolated from donors infected with ancestral SARS-CoV-2 viruses showed ultrapotent neutralization of emerging VOCs. The two most potent antibodies shared usage of the IGHV1-58 gene and targeted the RBD with minimal contact to VOC mutational hotspots. Cocktails of antibodies with complementary binding modes suppressed antibody escape.
” data-icon-position=”” data-hide-link-title=”0″>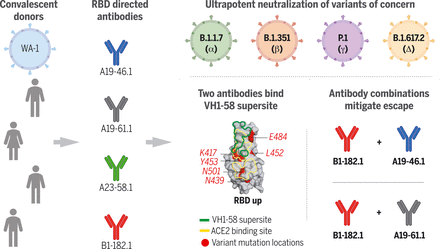
Antibodies isolated from donors infected with ancestral SARS-CoV-2 viruses showed ultrapotent neutralization of emerging VOCs. The two most potent antibodies shared usage of the IGHV1-58 gene and targeted the RBD with minimal contact to VOC mutational hotspots. Cocktails of antibodies with complementary binding modes suppressed antibody escape.
Abstract
The emergence of highly transmissible SARS-CoV-2 variants of concern (VOCs) that are resistant to therapeutic antibodies highlights the need for continuing discovery of broadly reactive antibodies. We identified four receptor binding domain–targeting antibodies from three early-outbreak convalescent donors with potent neutralizing activity against 23 variants, including the B.1.1.7, B.1.351, P.1, B.1.429, B.1.526, and B.1.617 VOCs. Two antibodies are ultrapotent, with subnanomolar neutralization titers [half-maximal inhibitory concentration (IC50) 0.3 to 11.1 nanograms per milliliter; IC80 1.5 to 34.5 nanograms per milliliter). We define the structural and functional determinants of binding for all four VOC-targeting antibodies and show that combinations of two antibodies decrease the in vitro generation of escape mutants, suggesting their potential in mitigating resistance development.
Since the start of the severe acute respiratory syndrone coronavirus 2 (SARS-CoV-2) outbreak, >170 million people have been infected, and >3.7 million have died from COVID-19 (1). The virus is decorated with a trimeric spike protein (S), which comprises an S1 subunit that binds host cells and an S2 subunit that is responsible for membrane fusion. The S1 subunit comprises an N-terminal domain (NTD); the receptor binding domain (RBD) that binds the host angiotensin-converting enzyme 2 (ACE2) receptor; and two additional subdomains, SD1 and SD2. Shortly after the first Wuhan Hu-1 (Hu-1) genome sequence was published (2), S proteins based on this sequence were generated for use in antibody discovery (3–5). SARS-CoV-2 variants such as B.1.1.7 (for example, Alpha, 501Y.V1) (6), B.1.351 (for example, Beta, 501Y.V2) (7), P.1 (for example, Gamma, 501Y.V3), and B.1.617.2 (for example, Delta, 452R.V3) (8, 9) contain mutations, many in S, that mediate resistance to therapeutic monoclonal antibodies, have increased transmissibility, and potentially increase pathogenicity (10–14). Vaccines designs based on the original Hu-1 outbreak strain sequence elicit antibody responses that show decreased in vitro neutralizing activity against variants (14–16). In this study, antibodies isolated from convalescent subjects who were infected by the Washington-1 (WA-1) strain, which has an identical S sequence to Hu-1, were investigated for reactivity against WA-1 and variants of concern (VOCs), and we defined the structural features of their binding to S.
Identification and characterization of antibodies against WA-1
We obtained blood from 22 convalescent subjects, who had experienced mild to moderate symptoms after WA-1 infection, between 25 and 55 days after symptom onset. Four subjects—A19, A20, A23, and B1—had both high neutralizing and binding activity against the WA-1 variant (Fig. 1A) and were selected for antibody isolation efforts. CD19+/CD20+/immunoglobulin M– (IgM–)/IgA+ or IgG+ B cells were sorted for binding to a stabilized version of S (S-2P), the full S1 subunit, or the RBD plus the subdomain-1 region of S1 (RBD-SD1) (Fig. 1B and fig. S1). In total, we sorted 889 B cells, recovered 709 (80%) paired heavy- and light-chain antibody sequences, and selected 200 antibodies for expression. A meso scale discovery (MSD) binding assay was used to measure binding of these 200 antibodies to stabilized spike, the full S1 subunit, RBD, or NTD. There was a broad response across all spike domains with 77 binding RBD, 46 binding NTD, 58 inferred to bind the S2 subunit based on binding to S but not to S1, and 19 binding an indeterminant epitope or failing to recognize spike in an MSD binding assay (Fig. 1C).
(A) Sera from 22 convalescent subjects were tested for neutralizing (y axis, ID50) and binding antibodies (x axis, S-2P ELISA AUC), and four subjects—A19, A20, A23, and B1 (colored) with both high neutralizing and binding activity against the WA-1—were selected for antibody isolation. (B) Final flow cytometry sorting gate of CD19+/CD20+/IgG+ or IgA+ PBMCs for four convalescent subjects (A19, A20, A23, and B1). Shown is the staining for RBD-SD1 BV421, S1 BV786, and S-2P APC or Ax647. Cells were sorted by using indicated sorting gate (pink), and percent of positive cells that were either RBD-SD1-, S1-, or S-2P-– positive is shown for each subject. (C) Gross binding epitope distribution was determined by using an MSD-based ELISA testing against RBD, NTD, S1, S-2P, or HexaPro. S2 binding was inferred from S-2P or HexaPro binding without binding to other antigens. Indeterminant epitopes showed a mixed binding profile. Total number of antibodies (200) and absolute number of antibodies within each group is shown. (D) Neutralization curves by using WA-1 spike pseudotyped lentivirus and live virus neutralization assays to test the neutralization capacity of the indicated antibodies (n = 2 to 3 replicates). (E) Table showing antibody binding target, IC50 for pseudovirus and live virus neutralization, and Fab:S-2P binding kinetics (n = 2 replicates) for the indicated antibodies. (F) SPR-based epitope binning experiment. Competitor antibody (y axis) is bound to S-2P before incubation with the analyte antibody (x axis) as indicated, and percent competition range bins are shown as red (>75%), orange (60 to 75%), or white (<60%) (n = 2 replicates). Negative control antibody is anti-Ebola glycoprotein antibody mAb114 (37). (G) Competition of ACE2 binding. The indicated antibodies (y axis) complete binding of S-2P to soluble ACE2 protein by using biolayer interferometry [left column, percent competition (>75% shown as red, <60% as white)] or to cell surface–expressed ACE2 by using cell-surface staining (right column, EC50 at ng/ml shown). (H) Negative-stain 3D reconstructions of SARS-CoV-2 spike and Fab complexes. A19-46.1 and A19-61.1 bind to RBD in the down position, whereas A23-58.1 and B1-182.1 bind to RBD in the up position. Representative classes were shown with two Fabs bound, although stoichiometry at one to three Fabs was observed.
” data-icon-position=”” data-hide-link-title=”0″>
(A) Sera from 22 convalescent subjects were tested for neutralizing (y axis, ID50) and binding antibodies (x axis, S-2P ELISA AUC), and four subjects—A19, A20, A23, and B1 (colored) with both high neutralizing and binding activity against the WA-1—were selected for antibody isolation. (B) Final flow cytometry sorting gate of CD19+/CD20+/IgG+ or IgA+ PBMCs for four convalescent subjects (A19, A20, A23, and B1). Shown is the staining for RBD-SD1 BV421, S1 BV786, and S-2P APC or Ax647. Cells were sorted by using indicated sorting gate (pink), and percent of positive cells that were either RBD-SD1-, S1-, or S-2P-– positive is shown for each subject. (C) Gross binding epitope distribution was determined by using an MSD-based ELISA testing against RBD, NTD, S1, S-2P, or HexaPro. S2 binding was inferred from S-2P or HexaPro binding without binding to other antigens. Indeterminant epitopes showed a mixed binding profile. Total number of antibodies (200) and absolute number of antibodies within each group is shown. (D) Neutralization curves by using WA-1 spike pseudotyped lentivirus and live virus neutralization assays to test the neutralization capacity of the indicated antibodies (n = 2 to 3 replicates). (E) Table showing antibody binding target, IC50 for pseudovirus and live virus neutralization, and Fab:S-2P binding kinetics (n = 2 replicates) for the indicated antibodies. (F) SPR-based epitope binning experiment. Competitor antibody (y axis) is bound to S-2P before incubation with the analyte antibody (x axis) as indicated, and percent competition range bins are shown as red (>75%), orange (60 to 75%), or white (<60%) (n = 2 replicates). Negative control antibody is anti-Ebola glycoprotein antibody mAb114 (37). (G) Competition of ACE2 binding. The indicated antibodies (y axis) complete binding of S-2P to soluble ACE2 protein by using biolayer interferometry [left column, percent competition (>75% shown as red, <60% as white)] or to cell surface–expressed ACE2 by using cell-surface staining (right column, EC50 at ng/ml shown). (H) Negative-stain 3D reconstructions of SARS-CoV-2 spike and Fab complexes. A19-46.1 and A19-61.1 bind to RBD in the down position, whereas A23-58.1 and B1-182.1 bind to RBD in the up position. Representative classes were shown with two Fabs bound, although stoichiometry at one to three Fabs was observed.
Pseudovirus neutralization assays by using the WA-1 spike showed that four RBD targeting antibodies—A19-46.1, A19-61.1, A23-58.1, and B1-182.1 (table S1)—are especially potent [half-maximal inhibitory concentration (the concentration of an antibody required to inhibit virus entry by 50%) (IC50) 2.5 to 70.9 ng/ml] (Fig. 1, D and E). WA-1 live virus neutralization (17) revealed similar high potent neutralization by all four antibodies (IC50 2.1 to 4.8 ng/ml) (Fig. 1, D and E). All four antibody Fabs exhibited nanomolar affinity for SARS-CoV-2 S-2P (2.3 to 7.3 nM), which is consistent with their potent neutralization (Fig. 1E).
Antibodies targeting the RBD can be categorized into four general classes (classes I to IV) on the basis of competition with the ACE2 target cell receptor protein for binding to S and recognition of the up or down state of the three RBDs in S (18). LY-CoV555 is a therapeutic antibody that binds RBD in both the up and down states, blocks ACE2 binding, and is categorized as class II. However, despite potent activity against WA-1, VOCs have been reported to contain mutations that confer resistance to LY-CoV555 (14, 19, 20) and similarly binding antibodies. We therefore examined whether the epitopes targeted by the four high-potency antibodies were distinct from LY-CoV555. We used a surface plasmon resonance (SPR)–based competition binding assay to compare the binding profile of these antibodies to LY-CoV555. Although LY-CoV555 competed with A19-46.1, A19-61.1, A23-58.1, and B1-182.1 (and vice versa), their overall competition profiles were not the same. A23-58.1 and B1-182.1 exhibit similar binding profiles, and A19-61.1 and A19-46.1 likewise display a shared competition binding profile in our SPR assay. However, the latter two antibodies can be distinguished from each other owing to A19-61.1 competition with the class III antibody S309 (Fig. 1F) (21), which binds an epitope in RBD that is accessible in the up or down position but does not compete with ACE2 binding (18).
To determine whether the antibodies block ACE2 binding, we used biolayer interferometry ACE2-competition and cell-surface binding assays to show that all four antibodies prevent the binding of ACE2 to spike (Fig. 1G and fig. S2). This suggests that A19-46.1, A23-58.1, and B1-182.1 neutralize infection by directly blocking the interaction of RBD with ACE2 and would be classified as either class I (ACE2 blocking, binding RBD up only) or II (ACE2 blocking, binding RBD up or down) RBD antibodies (18). A19-61.1 competition with S309 and ACE2 binding suggests that it binds at least partly outside of the ACE2 binding motif but may sterically block ACE2 binding similar to the class III antibody REGN10987. To refine the classification of these antibodies, we performed negative-stain three-dimensional (3D) reconstruction and found that A19-46.1 and A19-61.1 bound near one another with all RBDs in the down position (Fig. 1H), which is consistent with them being class II and class III antibodies, respectively. Similarly, A23-58.1 and B1-182.1 bound to overlapping regions when RBDs are in the up position, suggesting that they are class I antibodies.
Antibody binding and neutralization against circulating variants
Because each donor subject was infected with a variant close to the ancestral WA-1, we evaluated antibody activity against recently emerged variants such as D614G, which has become the dominant variant across the world (22). Similar to LY-CoV555, neutralization potency was increased against D614G compared with WA-1, with the IC50 and IC80 of each experimental antibody 1.4- to 6.3-fold lower than that seen for the WA-1 (IC50 of 0.8 to 20.3 ng/ml and IC80 of 2.6 to 43.5 ng/ml) (Fig. 2, A and C, and fig. S3). [Single-letter abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr. In the mutants, other amino acids were substituted at certain locations; for example, D614G indicates that aspartic acid at position 614 was replaced by glycine.]
(A) Table showing domain and mutations relative to WA-1 for each of the 10 variants tested in (B) and (C). (B) Spike protein variants were expressed on the surface of HEK293 T cells, and binding to the indicated antibody was measured with flow cytometry. Data are shown as MFI normalized to the MFI for the same antibody against the D614G parental variant. Percent change is indicated by a color gradient from red (increased binding, Max 500%) to white (no change, 100%) to blue (no binding, 0%). (C) IC50 and IC80 values for the indicated antibodies against 10 variants shown in (A). Ranges are indicated with white (>10,000 ng/ml), light blue (>1000 to ≤10,000 ng/ml), yellow (>100 to ≤1000 ng/ml), orange (>50 to ≤100 ng/ml), red (>10 to ≤50 ng/ml), maroon (>1 to ≤10 ng/ml), and purple (≤1 ng/ml). (D) Location of spike protein variant mutations on the spike glycoprotein for B.1.1.7, B.1.351, B.1.429, P.1 v2, B.1.617.1, and B.1.617.2. P681 and V1176 are not resolved in the structure, and therefore their locations are not noted in B.1.1.7 and P.1 v2.
” data-icon-position=”” data-hide-link-title=”0″>
(A) Table showing domain and mutations relative to WA-1 for each of the 10 variants tested in (B) and (C). (B) Spike protein variants were expressed on the surface of HEK293 T cells, and binding to the indicated antibody was measured with flow cytometry. Data are shown as MFI normalized to the MFI for the same antibody against the D614G parental variant. Percent change is indicated by a color gradient from red (increased binding, Max 500%) to white (no change, 100%) to blue (no binding, 0%). (C) IC50 and IC80 values for the indicated antibodies against 10 variants shown in (A). Ranges are indicated with white (>10,000 ng/ml), light blue (>1000 to ≤10,000 ng/ml), yellow (>100 to ≤1000 ng/ml), orange (>50 to ≤100 ng/ml), red (>10 to ≤50 ng/ml), maroon (>1 to ≤10 ng/ml), and purple (≤1 ng/ml). (D) Location of spike protein variant mutations on the spike glycoprotein for B.1.1.7, B.1.351, B.1.429, P.1 v2, B.1.617.1, and B.1.617.2. P681 and V1176 are not resolved in the structure, and therefore their locations are not noted in B.1.1.7 and P.1 v2.
Next, we assessed antibody binding to D614G and nine additional cell surface–expressed spike variants that have appeared subsequent to WA-1 and that are not considered VOCs or variants of interest (VOIs) (B.1.1.7.14, B.1.258.24, Y453F/D614G, Ap.1, B.1.388, ΔH69-70/N501Y/D614G, K417N/D614G, B.1.1.345, and B.1.77.31) (6–9, 22). Experimental antibodies were compared with four antibodies that are in clinical use [LY-CoV555, REGN10933, REGN10987, and CB6 (LY-CoV016)]. All control and experimental antibodies showed a minor reduction in binding (less than twofold) to B.1.258.24 (N439K/D614G) (figs. S3 and S4). Despite this, their neutralization capacities were minimally affected, with the exception of REGN10987 (2005 ng/ml) as reported previously (figs. S3 and S4) (23). Whereas none of the experimental antibodies showed large reductions in binding, LY-CoV555, CB6 (24), and REGN10933 (25) each showed >10-fold binding deficits to one or more variants (Y453F/D614G, K417N/D614G, B.1.1.345, or B.1.177.31) in these cell-based binding assays (figs. S3 and S4).
We next evaluated the capacity of each antibody to neutralize lentiviral particles pseudotyped with the same 10 variant spike proteins. Consistent with published data, REGN10933 did not neutralize Y453F/D614G or B.1.177.31 (K417N/E484K/N501Y/D614G) (12, 14, 26); CB6 did not neutralize B.1.177.31; and LY-CoV555 and REGN109333 showed potency reductions of 28- to >1400-fold for neutralization of viruses containing E484K (fig. S3) (12, 14). Relative to WA-1, the A23-58.1 IC50 neutralization was threefold lower for ΔH69-70/N501Y/D614G (0.9 ng/ml) and fivefold lower for Ap.1 (<0.6 ng/ml), and although A23-58.1 maintained high potency, neutralization against B.1.1.345 was increased fourfold (10.2 ng/ml). Neutralization by B1-182.1 maintained high potency (IC50 < 3.2 ng/ml) for all variants and showed more than fourfold improved potency for 6 of the 10 variants tested (IC50 < 0.8 ng/ml) (fig. S3). For A19-61.1, variant neutralization was three- to sixfold more potent than that of WA-1 (WA-1 IC50 70.9 ng/ml; variants IC50 11.1 to 23.7 ng/ml) (fig. S3). Last, neutralization by A19-46.1 was similar to that of WA-1 for all variants except B.1.1.345 and B.1.177.31, which were still highly potent despite having IC50 values that were two to threefold less active (B.1.1.345, 95.0 ng/ml; B.1.177.311, 61.8 ng/ml; and WA-1, 39.8 ng/ml) (fig. S3). Together, these data show the capacity of these newly identified antibodies to maintain high neutralization potency against a diverse panel of 10 variant spike proteins.
Antibody binding and neutralization of VOIs and VOCs
We analyzed neutralization of 13 circulating VOIs and VOCs, some of which have high transmissibility, including B.1.1.7, B.1.351, B.1.427, B.1.429, B.1.526, P.1, P.2, B.1.617.1, and B.1.617.2 (Fig. 2 and fig. S3) (6, 7, 11). Consistent with published data, we found that LY-CoV555, CB6, REGN10933, and REGN10987 maintained high potency against B.1.1.7 (IC50 0.1 to 40.1 ng/ml), and LY-CoV555 and CB6 were unable to neutralize B.1.351 v1, B.1.351 v2, P.1 v1, or P.1 v2 variants (IC50 > 10,000 ng/ml) (Fig. 2 and fig. S3) (12, 14, 26); LY-CoV555 was unable to neutralize B.1.526 v2, B.1.617.1, and B.1.617.2; CB6 showed 5- to 27-fold worse activity against B.1.1.7+E484K and B.1.429+E484K but remained active against B.1.617.1 and B.1.617.2; REGN10933 showed 9- to 200-fold reduction in neutralization against variants with mutations at E484 (B.1.1.7+E484K, B.1.429+E484K, B.1.526 v2, P.1 v1/v2, and B.1.617.1) and maintained activity against B.1.617.2, which does not contain a mutation at E484 (Fig. 2 and fig. S3); and REGN10987 maintained or had slightly increased potency against each of the VOCs and VOIs except B.1.617.2, which showed a fourfold reduction in activity (Fig. 2 and fig. S3). In comparison, A23-58.1, B1-182.1, A19-46.1, and A19-61.1 maintained similar or improved potency (IC50 < 0.6 to 11.5 ng/ml) against B.1.1.7 and B.1.1.7+E484K relative to WA-1 (Fig. 2 and fig. S3). The potency of A19-46.1 was within 2.5-fold or lower relative to WA-1 for all variants (IC50 11.5 to 101.4 ng/ml versus WA-1 39.8 ng/ml), except those containing L452R (IC50 >10,000 ng/ml) (B.1.427, B.1.429, B.1.429+E484K, B.1.617.1, and B.1.617.2) (Fig. 2 and fig. S3). Further analyses showed that A23-58.1, B1-182.1, and A19-61.1 maintained high potency against all VOCs and VOIs (IC50 < 0.6 to 28.3 ng/ml), including the recently identified B.1.617.1 and B.1.617.2 (Fig. 2 and fig. S3). These results indicate that despite being isolated from subjects infected with early ancestral SARS-CoV-2 viruses, each of these antibodies have highly potent reactivity against VOCs.
Structural and functional analysis of VH1-58 antibodies
The two most potent antibodies, A23-58.1 and B1-182.1, shared highly similar gene family usage in their heavy and light chains, despite being from different donors (table S1). Both use IGHV1-58 heavy chains and IGKV3-20/IGKJ1 light chains and similarly low levels of somatic hypermutation (SHM) (<0.7%) (table S1). This antibody gene family combination has been identified in other COVID-19 convalescent subjects and has been proposed as a public clonotype (27–30). To gain structural insights on the interaction between this class of antibodies and the SARS-CoV-2 spike, we obtained cryo–electron microscopy (cryo-EM) reconstructions for structures of the Fab A23-58.1 bound to a stabilized WA-1 S at 3.39 Å resolution and of the Fab B1-182.1 bound to a stabilized WA-1 S at 3.15 Å resolution (Fig. 3, A and B; figs. S5 and S6; and table S2). This revealed that the antibody bound to spike with all RBDs in the up position, confirming the negative stain results (Fig. 1H). However, the cryo-EM reconstruction densities of the interface between RBD and Fab were poor owing to conformational variation.
(A) Cryo-EM structure of A23-58.1 Fab in complex with SARS-CoV-2 HexaPro spike. (Left) Overall density map. Protomers are light green, gray, and cyan. One of the A23-58.1 Fab bound to the RBD is shown in orange and blue. (Right) Structure of the RBD and A23-58.1 after local focused refinement. The heavy-chain CDRs are brown, salmon, and orange for CDR H1, CDR H2, and CDR H3, respectively. The light-chain CDRs are marine blue, light blue, and purple blue for CDR L1, CDR L2, and CDR L3, respectively. The contour level of the cryo-EM map is 5.7σ. (B) Cryo-EM structure of B1-182.1 Fab in complex with SARS-CoV-2 HexaPro spike. (Left) Overall density map. Protomers are light green, gray, and cyan. One of the B1-182.1 Fab bound to the RBD is shown in salmon and light blue. (Right) Structure of the RBD and B1-182.1 after local focused refinement. The heavy-chain CDRs are brown, deep salmon, and orange for CDR H1, CDR H2, and CDR H3, respectively. The light-chain CDRs are marine blue, slate, and purple blue for CDR L1, CDR L2, and CDR L3, respectively. The contour level of the cryo-EM map is 4.0σ. (C) Interaction between A23-58.1 and RBD. All CDRs were involved in binding of RBD. Epitope of A23-58.1 is shown in bright green surface. RBD mutations in current circulating SARS-CoV-2 variants are red. K417 and E484 are located at the edge of the epitope. (D) Interaction details at the antibody-RBD interface. The tip of the RBD binds to a crater formed by the CDRs (shown viewing down to the crater). Interactions between aromatic and hydrophobic residues are prominent at the lower part of the crater. Hydrogen bonds at the rim of the crater are indicated with dashed lines. RBD residues are indicated with italicized font. (E) Paratopes of A23-58.1, B1-182.1, S2E12 (PDB ID: 7K45), and COVOX253 (PDB ID: 7BEN) from the same germline. Sequences of B1-182.1, S2E12, and COVOX253 were aligned with variant residues underlined. Paratope residues for A23-58.1, B1-182.1, S2E12, and COVOX253 were highlighted in green, dark green, light brown, and light orange, respectively.
” data-icon-position=”” data-hide-link-title=”0″>
(A) Cryo-EM structure of A23-58.1 Fab in complex with SARS-CoV-2 HexaPro spike. (Left) Overall density map. Protomers are light green, gray, and cyan. One of the A23-58.1 Fab bound to the RBD is shown in orange and blue. (Right) Structure of the RBD and A23-58.1 after local focused refinement. The heavy-chain CDRs are brown, salmon, and orange for CDR H1, CDR H2, and CDR H3, respectively. The light-chain CDRs are marine blue, light blue, and purple blue for CDR L1, CDR L2, and CDR L3, respectively. The contour level of the cryo-EM map is 5.7σ. (B) Cryo-EM structure of B1-182.1 Fab in complex with SARS-CoV-2 HexaPro spike. (Left) Overall density map. Protomers are light green, gray, and cyan. One of the B1-182.1 Fab bound to the RBD is shown in salmon and light blue. (Right) Structure of the RBD and B1-182.1 after local focused refinement. The heavy-chain CDRs are brown, deep salmon, and orange for CDR H1, CDR H2, and CDR H3, respectively. The light-chain CDRs are marine blue, slate, and purple blue for CDR L1, CDR L2, and CDR L3, respectively. The contour level of the cryo-EM map is 4.0σ. (C) Interaction between A23-58.1 and RBD. All CDRs were involved in binding of RBD. Epitope of A23-58.1 is shown in bright green surface. RBD mutations in current circulating SARS-CoV-2 variants are red. K417 and E484 are located at the edge of the epitope. (D) Interaction details at the antibody-RBD interface. The tip of the RBD binds to a crater formed by the CDRs (shown viewing down to the crater). Interactions between aromatic and hydrophobic residues are prominent at the lower part of the crater. Hydrogen bonds at the rim of the crater are indicated with dashed lines. RBD residues are indicated with italicized font. (E) Paratopes of A23-58.1, B1-182.1, S2E12 (PDB ID: 7K45), and COVOX253 (PDB ID: 7BEN) from the same germline. Sequences of B1-182.1, S2E12, and COVOX253 were aligned with variant residues underlined. Paratope residues for A23-58.1, B1-182.1, S2E12, and COVOX253 were highlighted in green, dark green, light brown, and light orange, respectively.
To resolve the antibody-antigen interface, we performed local refinement and improved the local resolution to 3.89 Å for A23-58.1 and to 3.71 Å for B1-182.1 (figs. S5 and S6). Because both A23-58.1 and B1-182.1 recognized the RBD in a very similar way, we used the RBD-A23-58.1 structure for detailed analysis. Antibody A23-58.1 binds to an epitope on the RBD that faces the threefold axis of the spike and is accessible only in the RBD-up conformation (Fig. 3A). The interaction buried a total of 619 Å2 surface area from the antibody and 624 Å2 from the spike (table S3). The A23-58.1 paratope constituted all six complementarity-determining regions (CDRs) with heavy chain and light chain contributing 74 and 26% of the binding surface area, respectively (Fig. 3, C and E, and table S3). The 14-residue-long CDR H3, which is 48% of the heavy-chain paratope, kinks at Pro95 and Phe100F (Kabat numbering scheme for antibody residues) to form a foot-like loop that is stabilized by an intraloop disulfide bond between Cys97 and Cys100B at the arch. A glycan was observed at the CDR H3 Asn96 (fig. S5F). The CDRs formed an interfacial crater with a depth of ~10 Å and a diameter of ~20 Å at the opening. Paratope residues inside the crater were primarily aromatic or hydrophobic. CDR H3 Pro95 and Phe100F lined the bottom, and CDR H1 Ala33, CDR H2 Trp50 and Val52, and CDR H3 Val100A lined the heavy-chain side of the crater (Fig. 3, D and E). On the light-chain side, CDR L1 Tyr32 and CDR L3 residues Tyr91 and Trp96 provided 80% of the light chain–binding surface (Fig. 3, D and E). By contrast, paratope residues at the rim of the crater are mainly hydrophilic; for example, Asp100D formed hydrogen bonds with Ser477 and Asn487 of the RBD (Fig. 3D and table S3).
The A23-58.1 epitope comprised residues between β5 and β6 at the tip of RBD (Figs. 3D and 4A). With the protruding Phe486 dipping into the crater formed by the CDRs, these residues formed a hook-like motif that is stabilized by an intraloop disulfide bond between Cys480 and Cys488. Aromatic residues—including Phe456, Tyr473, Phe486, and Tyr489—provided 48% (299 Å2) of the epitope (Fig. 3D and table S3). Lys417 and Glu484, which are located at the outer edge of the epitope, contributed only 3.7% of the binding surface (Fig. 3C and table S3). Overall, the cryo-EM analysis provides a structural basis for the potent neutralization of the E484K/Q mutant by A23-58.1.
(A) Mapping of epitopes of A23-58.1, B1-182.1, and other antibodies on RBD. Epitope residues for different RBD-targeting antibodies are marked with an asterisk under the RBD sequence. (B) Comparison of binding modes of A23-58.1 and B1-182.1. (Left) Analysis indicated that axis of Fab B1-182.1 is rotated 6° from that of A23-58.1. (Right) This rotation resulted in a slight shift of the epitope of B1-182.1 on RBD, which reduced its contact to E484. RBD mutations of concern are red, the epitope surface of B1-182.1 is dark green, and the borders of ACE2-binding site and A23-58.1 epitope are yellow and olive, respectively. (C) Comparison of binding modes of A23-58.1, CB6, and REGN10933. For clarity, one Fab is shown to bind to the RBD on the spike. The shift of the binding site to the saddle of RBD encircled K417, E484, and Y453 inside the CB6 (black line) and REGN10933 epitopes (violet surface), explaining their sensitivity to the K417N, Y453F, and E484K mutations. (D) Comparison of binding modes of A23-58.1 and LY-CoV555. (Left) One Fab is shown to bind to the RBD on the spike. (Top right) E484 is located inside the LY-CoV555 epitope. (Bottom right) E484K/Q mutation abolishes critical contacts between RBD and CDR H2 and CDR L3; moreover, E484K/Q and L452R cause potential clashes with heavy chain of LY-CoV555, explaining its sensitivity to the E484K/Q and L452R mutations. (E) IGHV1-58–derived antibodies target a supersite with minimal contacts to mutational hotspots. Supersite defined by common atoms contacted by the IGHV1-58–derived antibodies (A23-58.1, B1-182.1, S2E12, and COVOX253) on RBD is indicated with the green line. Boundaries of the ACE2-binding site and epitopes of class I, II, and III antibodies represented by C102 (PDB ID 7K8M), C144 (PDB ID 7K90), and C135 (PDB ID 7K8Z) are indicated with yellow, pink, light orange, and blue boundary lines, respectively.
” data-icon-position=”” data-hide-link-title=”0″>

