Although first detected in December 2019, COVID-19 was inferred to be present in Hubei province, China, for about a month before (1). Where did this new human disease come from? To understand the origin of the COVID-19 pandemic, it is necessary to go back to 2002. At that time a novel respiratory coronavirus appeared in Foshan, Guangdong province, China, and spread to 29 countries (2). Altogether ∼8000 people were infected with severe acute respiratory syndrome coronavirus (SARS-CoV) before public health measures controlled its spread in 2003. The zoonotic origin of SARS-CoV was subsequently linked to live animals available at markets. Further sporadic spill-over events of SARS-CoV from animals took place in Guangzhou, Guangdong, and some researchers working with cultured virus were infected in laboratory accidents (3), but ultimately SARS-CoV was removed from the human population. Trading of susceptible host animals is an important common theme in the emergence of SARS and COVID-19.
Three years after the SARS epidemic began, investigations revealed that horseshoe bats (Rhinolophus) in China were harboring related coronaviruses (4). These collectively form the species SARS-related coronavirus (SARSr-CoV), which comprises the Sarbecovirus subgenus of the Betacoronavirus genus. It was inferred that a sarbecovirus circulating in horseshoe bats seeded the progenitor of SARS-CoV in an intermediate animal host, most probably civet cats (3). Although other possible intermediate hosts for SARS-CoV were identified, in particular raccoon dogs and badgers (for sale with civet cats in animal markets), it is a population of civet cats within markets that appear to have acted as the conduits of transmission to humans from the horseshoe bat reservoir of SARS-CoV, rather than civet cats being a long-term reservoir host species. Presumably a captive civet cat initially became infected by direct contact with bats—e.g., as a result of bats foraging in farms or markets—or was infected prior to capture. Following the SARS epidemic, further surveillance revealed the immediate threat posed by sarbecoviruses from horseshoe bats. Despite this clear warning, another member of the SARSr-CoV species, SARS-CoV-2, emerged in 2019 that spread with unprecedented efficiency among humans. There has been speculation that the Wuhan Institute of Virology (WIV) in Hubei was the source of the pandemic because no SARS-CoV-2 intermediate host has been identified to date and owing to the WIV’s geographic location.
SARS-CoV-2 first emerged in Wuhan city, which is >1500 km from the closest known naturally occurring sarbecovirus collected from horseshoe bats in Yunnan province, leading to an apparent puzzle: How did SARS-CoV-2 arrive in Wuhan? Since its emergence, sampling has revealed that coronaviruses genetically close to SARS-CoV-2 are circulating in horseshoe bats, which are dispersed widely from East to West China, and in Southeast Asia and Japan (5). The wide geographic ranges of the potential reservoir hosts—for example, intermediate (R. affinis) or least (R. pusillus) horseshoe bat species, which are known to be infected with sarbecoviruses—indicate that the singular focus on Yunnan is misplaced (5). Confirming this assertion, the evolutionarily closest bat sarbecoviruses are estimated to share a common ancestor with SARS-CoV-2 at least 40 years ago (5), showing that these Yunnan-collected viruses are highly divergent from the SARS-CoV-2 progenitor. The first of these viruses reported by WIV, RaTG13 (6), is certainly too divergent to be the SARS-CoV-2 progenitor, providing key genetic evidence that weakens the “lab-leak” notion. Additionally, three other sarbecoviruses collected in Yunnan independently of the WIV are now the closest bat coronaviruses to SARS-CoV-2 that have been identified: RmYN02, RpYN06, and PrC31 (see the figure).
Coronaviruses that are evolutionarily closest to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been sampled in China, Cambodia, Japan, and Thailand (5). The phylogenetic tree, inferred from a genomic region minimized for recombination (5), shows sarbecoviruses closely related to SARS-CoV-2. Host species for each virus, horseshoe bat (Rhinolophus), human (Homo sapiens), and pangolin (Manis javanica) and the year of sample collection are shown in the key. Longquan140 is inferred from another genomic region (5) (dashed line). See supplementary table S1 for more details.
GRAPHIC: K. FRANKLIN/SCIENCE
” data-icon-position=”” data-hide-link-title=”0″>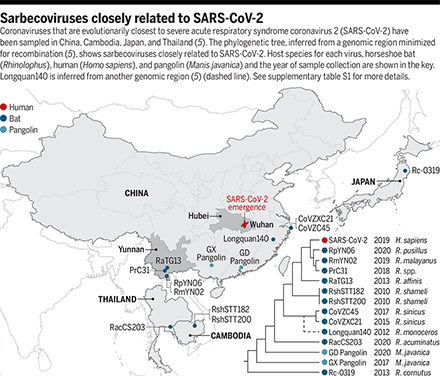

Coronaviruses that are evolutionarily closest to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been sampled in China, Cambodia, Japan, and Thailand (5). The phylogenetic tree, inferred from a genomic region minimized for recombination (5), shows sarbecoviruses closely related to SARS-CoV-2. Host species for each virus, horseshoe bat (Rhinolophus), human (Homo sapiens), and pangolin (Manis javanica) and the year of sample collection are shown in the key. Longquan140 is inferred from another genomic region (5) (dashed line). See supplementary table S1 for more details.
GRAPHIC: K. FRANKLIN/SCIENCE
So, how did SARS-CoV-2 get into humans? Although it is possible that a virus spillover occurred through direct horseshoe bat–to–human contact, a known risk for SARSr-CoVs (7), the first detected SARS-CoV-2 cases in December 2019 are associated with Wuhan wet markets (8). This is consistent with multiple animal-market–associated spillover events in November and December (9). It is currently not possible to be certain of the animal source of SARS-CoV-2, but it is notable that live animals, including civet cats, foxes, minks, and raccoon dogs, all susceptible to sarbecoviruses, were for sale in Wuhan markets, including the Huanan market (identified as an epicenter of the outbreak in Wuhan) throughout 2019 (10). Many of these animals are farmed for their fur at large scale and then sold to animal markets (11). Some of these farmed species (American minks, red foxes, and raccoon dogs) were sold alive for food by Wuhan animal sellers, as was trapped wildlife (including raccoon dogs and badgers), although no bat species were for sale (10). Together, this suggests a central role for SARSr-CoV–susceptible live intermediate host animals as the primary source of the SARS-CoV-2 progenitor that humans were exposed to, as was the case with the origin of SARS.
If these routes of transmission to humans are in place, why is emergence so rare that only two major outbreaks have occurred in the last two decades? Spillover events are not so unusual in locations where more frequent human-animal contacts take place. This is indicated by serology studies showing evidence for SARSr-CoV–specific antibodies in people living in rural locations (12), and even higher rates recorded in people living near bat caves (7). Spillover risk will increase with human encroachment into rural areas, resulting from new travel networks around and between urban areas. When a novel virus is then exposed to a densely packed human population, such as in Wuhan city, these spillover events have a much higher chance of resulting in substantial onward spread (1).
One particular ecological event in China that severely disrupted meat trade, and thereby contributed to increased wildlife–human contacts, was the shortage of pork products in 2019. This was a direct consequence of the African swine fever virus (ASFV) pandemic (11), which led to ∼150 million pigs being culled in China, resulting in a pork supply reduction of ∼11.5 million metric tons in 2019. Although production of other meat, such as poultry, beef, and fish products, moderately increased and China imported more of these products from international markets to mitigate the short-fall, this supply only covered a fraction of the ASFV-associated pork losses. Consequently, pork prices hit a record high in November 2019, with the wholesale price increasing ∼2.3 times compared with the previous year. Moreover, pig production has been relocating from Southern to Northern China since 2016. This, coupled with tight restrictions on the movement of live pigs and pork products to mitigate the ASFV pandemic, reduced the availability of pork in the Eastern and Southern provinces, resulting in much steeper price increases in these regions. In response, food consumers and producers may have resorted to alternative meats, including farmed or captured wildlife, especially in Southern China where wildlife is traditionally consumed (11). The resulting increased trade of susceptible farmed animals and wildlife could have brought humans into more frequent contact with meat products and animals infected with zoonotic pathogens, including SARSr-CoVs.
There are controversial reports of human SARS-CoV-2 cases in China being traced back to contact with imported frozen foods and SARS-CoV-2 apparently identified from frozen food, packaging, and storage surfaces (13). In an effort to prevent ASFV spread through live pig transportation routes, supply through the cold chain has been encouraged by the Chinese government since October 2018, with stronger support since September 2019 in the form of waiving freeway toll fees for frozen pork. The large demand for pork meat facilitated the use of cold-chain transport for all meat types, in particular from places with lower prices to those with higher prices, legally (or illegally), potentially also including transport of species susceptible to SARSr-CoV infection. The World Health Organization (WHO) Origins Report (8) recorded carcasses of wildlife, particularly badgers, left behind in freezers at the Huanan market, as well as their sale as frozen goods in late December 2019. It is likely that this wildlife had been trapped or farmed elsewhere and sold to Wuhan markets through the cold chain. Exposures could also potentially occur through feeding of coronavirus-infected carcasses to live animals either in transport or at markets.
The emergence of SARS-CoV-2 has properties that are consistent with a natural spillover (9). Although carriage from a bat cave of a sarbecovirus close enough to SARS-CoV-2 to be the progenitor as a research sample to the WIV is theoretically possible, such a scenario would be extremely unlikely relative to the scale of human-susceptible animal contacts routinely taking place in animal trading. Alternatively, bat guano (feces) is collected for use as fertilizer, again on a much larger scale than irregular research visits to bat caves, consistent with rare but ongoing SARSr-CoV transmissions to humans in rural areas (7, 12).
Overall, SARSr-CoV animal-to-human transmission associated with infected live animals is the most likely cause of the COVID-19 pandemic. However, the massive scale of cold-chain supply, particularly following disruption to the meat industry in China caused by ASFV-associated culling, suggests that frozen susceptible-animal carcasses, either for human or animal consumption, should not be discounted as playing a role in the emergence of SARS-CoV-2. This will especially be the case if the progenitor population of SARS-CoV-2 is found further away from Wuhan, because live-animal trafficking is much more likely to involve more proximal locations to the city, e.g., the prefectures of Hubei province. Serology, sampling and interviewing of the individuals (e.g., trappers, traders, and farmers) connected to the sources of wildlife sold in the Wuhan markets in October and November 2019 would be a sensible next step in future investigations.
Horseshoe bats, such as this greater horseshoe bat (Rhinolophus ferrumequinum), can be a reservoir of coronaviruses.
PHOTO: DIETMAR NILL/MINDEN PICTURES
Once in the human population, SARS-CoV-2 has spread surprisingly rapidly for a new human pathogen. Contrary to classical expectations for a host species jump, SARS-CoV-2 is highly capable of human transmission, including frequent asymptomatic transmission and amplification through superspreader events. This initial “success,” at least prior to the emergence of variants of concern, is unlikely to be due to early adaptation to humans but rather can be attributed to the relatively generalist nature of SARS-CoV-2 (14), evidenced by frequent transmission to mammals: minks, cats, and others. Worryingly, recent experimental evidence has found that the pangolin-derived sarbecoviruses (presumably acquired from exposure to horseshoe bats or other infected animals after illegal trafficking into China) can also infect human cells and have spike proteins that are even better at facilitating entry into human cells than that of SARS-CoV-2 (15). Collectively this points to a further risk of spillover that extends to the more divergent members of the lineage that SARS-CoV-2 emerged from and implies frequent spillovers from bats to other susceptible wildlife.
Humans are now the dominant SARS-CoV-2 host species. The danger is that SARS-CoV-2 could spread from humans to other animal species, termed reverse zoonosis, as is suspected for white-tailed deer in the United States. The promiscuous infection of various host species by the sarbecoviruses means that future spillovers of SARSr-CoVs from wildlife are very likely, and current vaccines may not be protective against novel variants. The sampling intensity of sarbecoviruses needs to be urgently increased to gain a better understanding of this spillover risk. The recent finding of sarbecoviruses, not dissimilar to SARS-CoV-2, dispersed in Southeast Asia emphasizes the urgency of monitoring coronavirus diversity. Humanity must work together beyond country borders to amplify surveillance for coronaviruses at the human–animal interface to minimize the threat of both established and evolving variants evading vaccines and to stop future spillover events.
Acknowledgments: We thank the researchers who have shared genome data openly via GenBank or GISAID. D.L.R. and J.H. are funded by the Medical Research Council (MRC) (MC_UU_1201412) and D.L.R. by the Wellcome Trust (220977/Z/20/Z). S.L. is funded by an MRC studentship.

