The patient arrived at the hospital one hot night in Masi-Manimba, an agricultural town unfurled along the Democratic Republic of the Congo’s Lukula River.
He couldn’t speak, he couldn’t walk, he was conscious but “barely could make … gestures,” says Béatrice Kasita, a nurse who was there when he came in. She remembers his deformed posture, how his body curved into a fetal position.
He was also unusually drowsy — a telltale sign of his illness. The patient, a 27-year-old man, had been brought in by a medical team screening villagers for sleeping sickness, a deadly parasitic disease spread via the bite of a blood-feeding fly.
Science News headlines, in your inbox
Headlines and summaries of the latest Science News articles, delivered to your email inbox every Thursday.
Thank you for signing up!
There was a problem signing you up.
Since the first case report in the late 14th century, the illness has ebbed and flowed in sub-Saharan Africa. Across the continent, the predominant form of sleeping sickness shows up in about two dozen countries, most cases now occurring in the DRC. The disease is a nightmarish scourge that can maim the brain and ultimately kill. But today, cases hover near an all-time low. In 2021, the World Health Organization reported just 747 cases of the predominant form, down from more than 37,000 in 1998.
That precipitous plunge came out of decades of work, millions of screenings, spinal taps upon spinal taps, toxic treatments and the rapid rise of safer though often burdensome ones, countless IV infusions, long hospital days and nights, medicine lugged to remote villages, and communities on constant alert for sleeping sickness’s insidious symptoms.
Now, a promising drug has fanned hope for halting transmission of the disease. Called acoziborole, the drug is taken by mouth in just a single dose. Kasita’s patient, who arrived at the hospital in June 2017, was among the first to try it.
Her hospital is one of 10 clinical trial sites in the DRC and Guinea working to test the drug with the Drugs for Neglected Diseases initiative, or DNDi, a nonprofit organization based in Geneva. In a small trial reported last year, the drug appeared to be safe and effective. A larger trial is ongoing, with results expected by the end of this year.
If the findings hold up, the drug would be “a game changer,” says Emmanuel Bottieau, an infectious disease specialist at the Institute of Tropical Medicine in Antwerp, Belgium, who is not involved with the clinical trial. A single-dose medication is “really a dream for us, coming from such a long history of very difficult or toxic or cumbersome treatments.”
But he and others know that even a game-changing drug doesn’t guarantee a win. The dominant form of sleeping sickness is on a short list of neglected tropical diseases the WHO is targeting for elimination by 2030. That means bringing cases in certain areas down to zero knowing that some control efforts may still be required. Vastly harder to achieve is disease eradication, where cases worldwide stay parked at zero permanently. (To date, just a single human infectious disease — smallpox — has been eradicated.)
Subscribe to Science News
Get great science journalism, from the most trusted source, delivered to your doorstep.
Even elimination is no easy task — and can get harder as you approach the finish line. “We are advancing very well,” says José Ramón Franco, a WHO medical officer based in Geneva, “but we [haven’t] reached the last mile.”
Still, tiptoeing along the edges of optimism, some, like Kasita, are finding moments to cheer. For the severely ill patient, her team initially wondered if acoziborole would work. “Are we really going to help him with this single-dose treatment?”
Two weeks later, he could stand, with some support, and had started speaking again, a radical recovery. Kasita smiles widely as she remembers it. Watching him heal “was a great pleasure,” she says.
The symptoms of sleeping sickness
About 400 kilometers to the west of Masi-Manimba, physician Wilfried Mutombo Kalonji is preparing to visit Kasita’s hospital. Afterward, he’ll hit up hospitals in Idiofa, Bagata and then Bandundu, three other acoziborole clinical trial sites in the DRC. To reach the sites, Mutombo will travel by boat, plane, car and motorbike. He’ll stay in both modern hotels and hotels without running water or electricity. Then, he’ll return home to Kinshasa, the DRC’s bustling capital. It’s a great and noisy city, he grins, with people playing music in the streets and “many, many, many traffic jams.”
In Kinshasa, Roi Baudouin hospital is one of the DNDi’s acoziborole trial sites. Mutombo has been organizing logistics and ensuring that each site has what it needs to treat and monitor patients. That includes generators for electricity, an internet connection, medical equipment and trained clinical trial staff.
Mutombo has worked with sleeping sickness patients since 2004. Two weeks after finishing his medical training in Kasaï province, he shipped out to Kasansa, becoming the only medical doctor in a village of about 11,000 people. In Kasansa, which lies in western DRC, north of the Angola border, sleeping sickness was then, and still remains, endemic.
The disease, also called human African trypanosomiasis, is caused by a single-celled, ruffle-edged parasite that worms its way into the brain. One subspecies, Trypanosoma brucei gambiense, causes the vast majority of cases and tends to plague western and central Africa. Another, T.b. rhodesiense occurs in the eastern and southern parts of the continent and causes a more rapid, acute illness with far fewer cases in people.
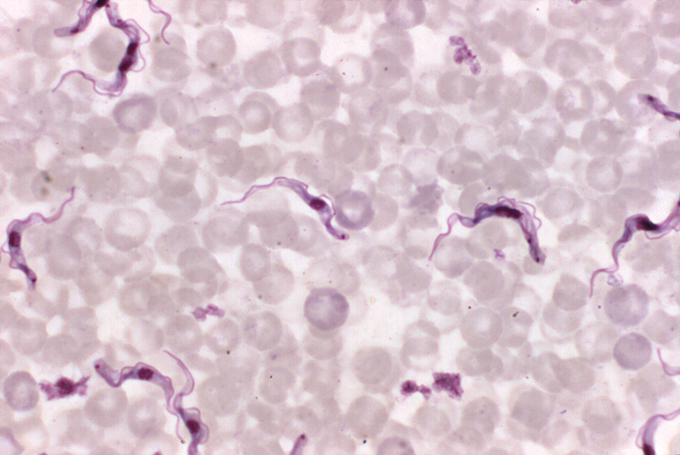
Both subspecies can ride in the guts and glands of tsetse flies, which often buzz near bodies of water; many of Mutombo’s patients in Kasansa were fishers or farmers. When the fly bites, the parasite enters the bloodstream. From there, it can get picked up again when other flies feed, shuttling from insects to humans in a disease-spreading cycle.
In the blood, T.b. gambiense sparks a slow-burning illness that can begin with a fever and, if left untreated, end with death. As the parasite multiplies, lymph nodes enlarge and the head, muscles and joints ache. Patients can also become intensely itchy, scratching hard enough to damage the skin, Kasita says.
When the parasite slips past the blood-brain barrier, patients enter the second stage of the disease. No one knows exactly where the parasite lodges in the brain, but neurological symptoms can vary. Doctors and nurses describe a range of distressing and bizarre behaviors. One common behavior gives the illness its name. Somehow, the parasite reverses people’s sleep/wake cycle. “They will sleep a lot during the day, and at night, they will be up, watching,” Kasita says.
Patients can also feel depressed and confused, neglect to care for themselves, hallucinate or experience logorrhea, words cascading from lips in nonsensical streams. In some infected people, personalities can swing like a wrecking ball. Jacques Pépin, an infectious disease specialist at the University of Sherbrooke in Canada, worked with sleeping sickness patients in the 1980s and remembers one who suddenly threw a large rock at his head.
Such outbursts can be scary for patients and families, says Antoine Tarral, a pharmacologist and infectious disease physician who works with Mutombo and led the DNDi’s sleeping sickness program for 10 years. Fear of the disease can prompt villages to reject infected individuals, he says.
Sleeping sickness carries a social stigma that makes people feel like outcasts, Mutombo agrees. “This disease is terrible.” When he first began treating patients, he says, “I was doing my best to make them feel like human beings.”
But for decades, available treatments were terrible, too.

Sleeping sickness has a history of terrible treatments
For most of treatment history, injected or intravenous drugs were the only option for sleeping sickness. They could cure patients, but only if doctors administered them in time. And when cases advanced to the second stage, medical staff had to switch tactics. For patients, that meant a spinal tap to confirm diagnosis followed by different drugs.
Until the late 2000s, the most-used treatment for advanced gambiense sleeping sickness was the highly toxic melarsoprol. The drug is derived from arsenic (and it’s still the leading treatment for advanced rhodesiense cases). Medical staff administered the drug for 10 days via daily intravenous infusions that burned entering patients’ veins, Mutombo says. The treatment could also be lethal, killing some 5 percent of patients.
Mutombo grows somber remembering two of his patients who died, young men he tried to cure in Kasansa. “That was a very bad experience,” he says. “When patients come to the hospital, they come to receive a treatment, not to die … [from] the drug we gave them.”
But doctors didn’t have a lot of options. Without melarsoprol, patients with serious cases faced near-certain death.
Not long after his patients died, Mutombo heard that the DNDi was launching a project on a new, less toxic treatment for advanced cases. He jumped at the chance, applied to be an investigator and joined the project in 2006. The new treatment, called NECT, combined eflornithine, an IV drug developed for cancer, with the oral drug nifurtimox. Eflornithine was already being used to treat sleeping sickness, but required dozens of infusions, and nifurtimox was a treatment for Chagas disease.
In 2009, after a clinical trial and the WHO’s endorsement, NECT took off, rocketing past melarsoprol or eflornithine alone as the first-line treatment for advanced sleeping sickness. But NECT had some logistical snafus, Mutombo says. It wasn’t easy to transport, for one. Treatment for four patients came in 40-kilogram packages that had to be trucked over bad roads into rural areas that lacked medical workers. “That was a problem with NECT,” Mutombo says. “It was effective, but it was heavy and needed trained staff.”
Less than a decade later, Mutombo, Tarral and their DNDi colleagues debuted an easier alternative. Fexinidazole, at long last, was a drug doctors could deliver exclusively via pills rather than an IV. It’s not perfect — it’s administered by a nurse, patients need to take it for 10 days and it’s not best for the most severe cases (for these, the WHO still recommends NECT). But easy-to-use oral drugs lower the burden on health systems, Mutombo says. Medical staff could more easily bring treatments to remote patients. And that brought scientists one step closer to sleeping sickness’s elimination.
A new drug could help bring cases to zero
Acoziborole, the drug now being tested in clinical trials, may be another big step in the right direction. Just one dose cured some 95 percent of patients with late-stage infections, Mutombo, Tarral and colleagues reported November 29 in the Lancet Infectious Diseases. That’s comparable to treatment with NECT. “Acoziborole is one solution to manage this disease,” Tarral says.
Not only does the drug seem to be effective, but “it’s given orally … and it needs to be given only once,” says the University of Sherbrooke’s Pépin, who was not involved with the trial but wrote an opinion piece that appeared alongside the new report.
Yet, as Pépin points out, the acoziborole study has some limitations. The scientists tested the drug in just 208 patients, so no one knows if serious adverse effects might occur in larger populations. And the study wasn’t performed like the classic gold-standard clinical trial, with patients randomly assigned into different groups receiving different interventions.
Tarral acknowledges these drawbacks, which he says were due to low participant numbers. The researchers included only people with video-confirmed parasitic infections, which required years of searching for patients across 10 hospitals in two different countries.
“It’s not the standard approach, but that was the only possible approach,” Pépin says. “They did what could be done with the number of cases that are occurring now.”
The study’s promising results spurred a new, larger trial that will include 1,200 participants. This time, the team is enrolling people with positive antibody blood tests even if the parasite’s presence hasn’t been confirmed. Many of these participants may not actually be sick, says Veerle Lejon, a scientist at the French National Research Institute for Sustainable Development in Montpellier who was not involved in developing the drug but is collaborating with the DNDi on evaluating sleeping sickness diagnostics.
What this trial will offer, she says, is a raft of new data that will help determine the drug’s safety.
The challenges of eliminating an infectious disease
Even if acoziborole gets the green light, stamping out sleeping sickness isn’t a sure bet.
Eliminating an infectious disease is a slippery task. Success can, paradoxically, churn out new challenges. When case numbers dip low enough, for instance, interest in the disease can wane. Donors move money to other public health priorities, and once-robust control programs wither.
That happened for sleeping sickness in the 1960s, the last time cases dropped. Over the next few decades, cases ratcheted up, and epidemics broke out in Angola, the DRC and South Sudan. “Control of the disease was neglected, and then slowly, the disease came back,” says WHO medical officer Franco.
A doubled-down effort to find cases and treat them with ever-improving drugs got sleeping sickness under control again, with case numbers cratering to their low point today. But that level of surveillance is not sustainable, Franco says.

Health care workers can also lose knowledge of how to recognize the disease as they encounter fewer and fewer infected individuals, says Jennifer Palmer, a medical anthropologist at the London School of Hygiene and Tropical Medicine. “The challenge is really in making sure that people are aware that sleeping sickness is still a problem,” she says. In a small study in South Sudan, reported in 2020, Palmer and colleagues found that lay people encouraging people in the community to get tested accounted for more than half of detected cases.
Still, getting patients tested and treated can depend on whether they’re able to safely travel to health facilities. With the threat of violence in South Sudan and armed conflict in eastern DRC, the fate of sleeping sickness may also be shaped by the whims of war.
Even if every infected person was promptly found and treated, the disease-causing parasite would likely linger in wild and domestic animals. Scientists have found T.b. gambiense, for instance, in dogs, pigs, goats and sheep. No one knows the role infected animals play in reigniting outbreaks in humans.
Though the road to elimination may still be rocky, the patients Kasita and others are treating in Masi-Manimba and beyond offer a lesson for those working on disease elimination: Don’t give up too soon. Maybe the world won’t reach zero sleeping sickness cases by 2030, Lejon says, “but I think we should really give it a try,” she says. “We have momentum at this moment to do it.”
Mutombo echoes her enthusiasm. In less than 20 years, new drugs have completely overhauled patient care, he says. “We’ve made a great change in less than one generation…. Now, we expect that elimination is within reach.”


