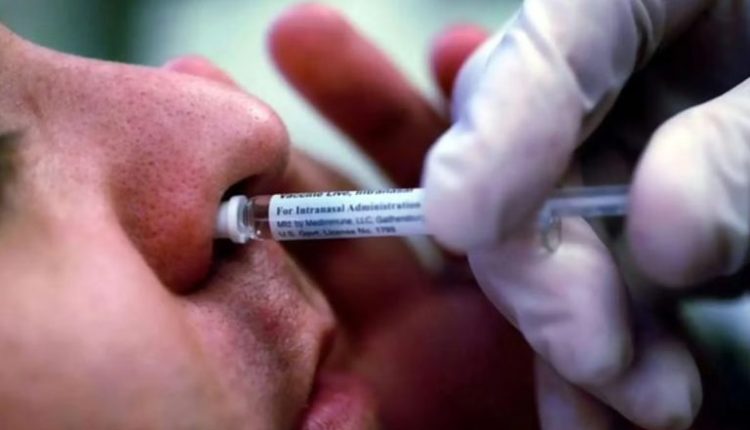
World’s first Nasal Vaccine for COVID-19 is set to be rolled out in the fourth week of January 2023 across India. Developed by Bharat Biotech, iNCOVACC (BBV154), recently received approval from the Central Drugs Standard Control Organization (CDSCO) to be used as a heterologous booster dose.
It will be administered as a booster dose for those above 18 years of age. The needle-free vaccine will be included in the CoWin app under India’s vaccination programme. As per the latest release by Bharat Biotech, the vaccine will be capped at Rs. 800 for Private Markets and Rs. 325 for supplies to Govt of India and State Governments, excluding GST on both. Nasal Vaccine Against COVID-19 Approved by Government of India, To Be Used As Heterologous Booster From Today.
This vaccine has the double benefit of enabling faster development of variant-specific vaccines and easy nasal delivery that enables mass immunization to protect from emerging variants of concern. Recognised as the “World’s first intra-nasal vaccine for COVID,” it promises to become an important tool in mass vaccinations during pandemics and endemics. World’s First Intra-Nasal Vaccine for Coronavirus Gets CDSCO’s Approval for Restricted Use.
Only for those who have not yet taken a precautionary dose
In an interview with Media, Dr NK Arora, Chairman of the Covid Working Group of NTAGI, said that the Nasal Vaccine is recommended as the first booster. It is not recommended for individuals who have already received a precautionary dose. “The vaccine is available for those who have not yet taken a precautionary dose,” he stated.
iNCOVACC® had earlier received approval from the Drugs Controller General for restricted use in an emergency situation (18 years +) for a primary 2-dose schedule as a heterologous booster dose.
“As part of the programme, CoWIN will not accept a fourth dose,” Covid Task Force Chief said. Stating reasons behind this he informed that ‘as per the concept of antigen sink, if a person is repeatedly immunised with a particular type of antigen, the body stops responding or responds poorly. This is why initially mRNA vaccines are given with a gap of six months. Later on, people are administered those in a three-month gap. “But since it has not helped too much in that case, at the moment taking a fourth dose is of no value,” he said.
Nasal Vaccine for cost-effectiveness in low & middle-income countries
“We have achieved the goals we set for ourselves during this pandemic. We have developed COVAXIN and iNCOVACC, two COVID vaccines from two different platforms, with two different delivery systems. The vectored intranasal delivery platform gives us the capability for rapid product development, scale-up, easy and painless immunization during public health emergencies and pandemics,” Dr. Krishna Ella, Executive Chairman remarked.
As a needleless vaccination, iNCOVACC provides the double benefit of enabling faster development of variant-specific vaccines and easy nasal delivery that enables mass immunization to protect from emerging variants of concern. Moreover, as per the statement of Dr Arora, this vaccine is not only going to help fight Covid but all respiratory viruses and infections.
iNCOVACC was developed in partnership with Washington University, St. Louis, which had designed and developed the recombinant adenoviral vectored construct and evaluated it in preclinical studies for efficacy. The Nasal vaccine candidate was evaluated in Phases I, II and III clinical trials with successful results. The nasal delivery system has been designed and developed for its efficiency and cost-effectiveness in low and middle-income countries.
(The above story first appeared on Today News 24 on Dec 28, 2022 08:50 PM IST. For more news and updates on politics, world, sports, entertainment and lifestyle, log on to our website todaynews24.top).