INTRODUCTION
As of January 2021, the recently emerged severe-acute respiratory syndrome coronavirus 2 has led to over 2 million deaths and over 100 million infections globally (1). SARS-CoV-2 is a member of the Coronaviridae family of viruses. Respiratory infections with SARS-CoV-2 can result in asymptomatic, mild or severe forms of a disease known as COVID-19. More severe cases of COVID-19 result in death due to acute respiratory distress syndrome and damage to the alveolar lumen (2). Currently, there are few treatment options for COVID-19 patients. The antiviral RNA-dependent polymerase inhibitor remdesivir reduces length of hospitalization and deaths from COVID-19 (3). In addition, the steroid dexamethasone has also been approved for use in severe COVID-19 (4). To date numerous efficacious vaccines have been developed and rolled out (5, 6). Despite these advances additional antiviral therapeutics will be required for the treatment of future endemic infections. An ongoing global effort is now underway to identify and develop new antiviral and anti-inflammatory therapeutics to reduce COVID-19 related hospitalizations and deaths.
The ER-resident adaptor protein Stimulator of Interferon Genes is a key signaling molecule that is activated following cytosolic DNA detection. Cyclic GMP-AMP synthase is an innate immune sensor of cytosolic DNA. Upon DNA binding cGAS converts ATP and GTP into the cyclic dinucleotide cGAMP, which in turn binds and activates STING (7). Conformational changes in STING lead to C-terminal phosphorylation of STING, dimerization, oligomerization and subsequent activation of autophagy, NF-κB, IRF3 and transcription of proinflammatory cytokines and type I IFNs (8–10). Activation of STING can elicit a potent anti-tumor response and the use of STING agonists in oncology alone or in combination with checkpoint blockade is an emerging therapeutic area (11–13). A recent study has identified a new class of STING agonists with systemic in vivo activity. Diamidobenzimidazole based compounds are potent, specific activators of STING and possess superior stability, tissue penetrance and potency over traditional cyclic dinucleotide STING agonists (14). While the therapeutic applications of STING agonists have been reported for use in oncology, the antiviral potential of STING agonists remains underexplored. Given the potent type I IFN response induced by diABZI compounds, we hypothesized that pharmacological activation of STING may elicit protection from SARS-CoV-2 infection.
RESULTS
diABZI-4 activates STING
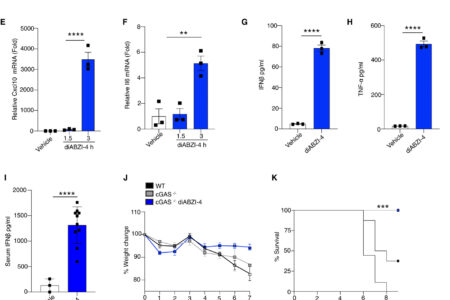
To evaluate the antiviral properties of diABZI compounds we utilized a new diABZI compound, diABZI-4 (Fig. 1A, supplementary methods). diABZI-4 has a more favorable solubility profile for in vivo studies in comparison to the previously reported diABZI-3 (14). diABZI-4 induced oligomerization of STING (Fig. 1B), transcription of IFNB1, CXCL10, TNF and IL6 (Fig. 1C-F) and the secretion of IFN-β (Fig. 1G) and TNF-α (Fig. 1H) in primary human CD14+ monocytes. Similarly, diABZI-4 potently activated STING in murine cells. Treatment of bone marrow derived macrophages (BMDMs) with diABZI-4 resulted in oligomerization of STING (Fig. S1A), phosphorylation of IRF3, TBK1, p65 and autophagy (LC3 conversion) (Fig. S1B). Treatment of BMDMs with diABZI-4 also induced the expression (Fig. S1C-F) and secretion (Fig. S1G-J) of IFN-β, CXCL10, TNF-α and IL-6. In vivo administration of diABZI-4 via intraperitoneal injection also induced the production of IFN-β (Fig. 1I).
diABZI-4 promotes potent STING activation and protection from HSE (A) Structure of diABZI-4. (B) Native immunoblot of STING dimerization in CD14+ human monocytes treated with 0.1 μM of diABZI-4 for the indicated times. (C-F) QPCR analysis of IFNβ (C), TNF-α (D), CXCL10 (E) and IL-6 (F) mRNA expression in CD14+ human monocytes treated with 0.1 μM of diABZI-4 for the indicated times. IFNβ (G) and TNF-α (H) ELISA in CD14+ human monocytes treated with 0.1 μM of diABZI-4 for the indicated times. (I) Serum IFNβ levels from WT mice 3 hours after intraperitoneal injection with 1mg/kg diABZI-4 (n=10) or PBS (n=3). (J-M) Weight loss (J) survival (K) hydrocephalus (L) and neurological symptom scores of WT (n=8) and cGAS−/− (n=7-8) mice infected in the cornea with 2×105 PFU of HSV-1 McKrae with a 1 hour retro-orbital pre-treatment of vehicle control or 0.5mg/kg of diABZI-4. C-H are pooled data from three independent experiments, error bars show means ± SEM. B, representative experiment. I, data points indicate individual mice. J-M, data points indicate mean ± SD of each group. **, P<0.01; ***, P<0.001; ****, P<0.0001 (B-F one way ANOVA. G-I Student’s t test. J, L-M 2-way ANOVA. K, Mantel-Cox).
” data-icon-position=”” data-hide-link-title=”0″>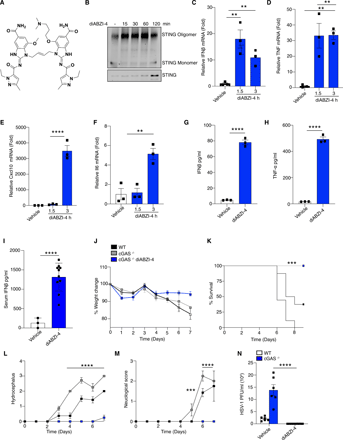
diABZI-4 promotes potent STING activation and protection from HSE (A) Structure of diABZI-4. (B) Native immunoblot of STING dimerization in CD14+ human monocytes treated with 0.1 μM of diABZI-4 for the indicated times. (C-F) QPCR analysis of IFNβ (C), TNF-α (D), CXCL10 (E) and IL-6 (F) mRNA expression in CD14+ human monocytes treated with 0.1 μM of diABZI-4 for the indicated times. IFNβ (G) and TNF-α (H) ELISA in CD14+ human monocytes treated with 0.1 μM of diABZI-4 for the indicated times. (I) Serum IFNβ levels from WT mice 3 hours after intraperitoneal injection with 1mg/kg diABZI-4 (n=10) or PBS (n=3). (J-M) Weight loss (J) survival (K) hydrocephalus (L) and neurological symptom scores of WT (n=8) and cGAS−/− (n=7-8) mice infected in the cornea with 2×105 PFU of HSV-1 McKrae with a 1 hour retro-orbital pre-treatment of vehicle control or 0.5mg/kg of diABZI-4. C-H are pooled data from three independent experiments, error bars show means ± SEM. B, representative experiment. I, data points indicate individual mice. J-M, data points indicate mean ± SD of each group. **, P<0.01; ***, P<0.001; ****, P<0.0001 (B-F one way ANOVA. G-I Student’s t test. J, L-M 2-way ANOVA. K, Mantel-Cox).
diABZI-4 protects against herpes simplex encephalitis
To evaluate the potential antiviral effects of systemically administered diABZI-4, we first tested the efficacy of diABZI-4 in a mouse model of herpes simplex encephalitis. cGAS- and STING-deficient mice are highly susceptible to acute HSV-1 infection (15). Indeed, both cGAS and STING-deficient mice displayed increased weight loss, hydrocephalus and decreased survival following corneal infection with a neuroinvasive strain of HSV-1 (Fig. S2A-F). Thus, we hypothesized that diABZI-4 may compensate for cGAS deficiency and confer protection from HSE through activation of STING. Treatment of cGAS−/− mice with a single dose of diABZI-4 delivered via retro-orbital injection resulted in complete protection from HSE (Fig. 1J-M). cGAS−/− mice receiving diABZI-4 were protected from HSV-1–induced weight loss (Fig. 1J), lethality (Fig. 1K), hydrocephalus (Fig. 1L) and symptoms of neurological disease (Fig. 1M). In addition, cGAS−/− mice receiving diABZI-4 had a significant reduction in HSV-1 titers in brain tissue (Fig. 1N).
diABZI-4 inhibits SARS-CoV-2 replication
Based on these findings we next assessed if diABZI-4 could also confer protection against RNA viruses. We focused on human coronavirus OC-43 (HCoV-OC43) and SARS-CoV-2. SARS-CoV-2 infects lung epithelial cells via the ACE2 receptor (16). Therefore, we first monitored STING activation in ACE2-expressing A549 cells treated with diABZI-4 which resulted in the time-dependent dimerization of STING (Fig. 2A), phosphorylation of IRF3 (Fig. 2B) and expression of IFNB1 (Fig. 2C) and TNF (Fig. 2D). ACE2-A549 cells were highly permissive to HCoV-OC43 and SARS-CoV-2 infection (2). Pre-treatment of ACE2-A549 cells with diABZI-4 prior to infection with HCoV-OC43 inhibited HCoV-OC43 replication when compared to vehicle-treated cells (Fig. 2E-F). Similarly, pre-treatment with diABZI-4 inhibited SARS-CoV-2 gene expression and replication in infected cells when compared to vehicle-treated cells (Fig. 2G-K). Given the strong effects diABZI-4 had on SARS-CoV-2 replication in hACE2-A549 cells we next evaluated its effects on SARS-CoV-2 in a more physiologically relevant lung alveolar cell system. Embryonic stem cell-derived induced alveolar type II (iAT2) cells were cultured in 3D at air-liquid interface (ALI). Pre-treatment of iAT2 cells with diABZI-4 inhibited SARS-CoV-2 replication. (Fig. 2L-M). Two other studies have also shown diABZI molecules impair SARS-CoV-2 replication in lung epithelial cells (17, 18)
diABZI-4 inhibits SARS-CoV-2 replication in lung epithelial cells. (A) Native immunoblot of STING dimerization from ACE-A549 cells treated with 0.1 μM of diABZI-4 for the indicated times. (B) Immunoblot analysis of p-IRF3, STING, ACE2 and β-actin from ACE2-A549 cells treated with 0.1 μM of diABZI-4 for the indicated times. (C-D) QPCR analysis of IFNβ (C) and TNF-α (D) mRNA expression in ACE2-A549 cells treated with 0.1 μM of diABZI-4 for 1.5 hours. (E) QPCR analysis of HCoV-OC43-N mRNA from ACE2-A549 cells infected with HCoV-OC43 0.1 MOI for 24 hours with or without pre-treatment of 0.1 μM diABZI-4. (F) TCID50 assay of conditioned medium from ACE2-A549 cells treated as in E for 48 hours. (G-I) QPCR analysis of SARS-CoV-2-N (G), Nsp14 (H) and ORF1 (I) mRNA from ACE2-A549 cells infected with SARS-CoV-2 0.1 MOI for 24 hours with or without pre-treatment of 0.1 μM diABZI-4. (J) Genome copy number analysis on conditioned medium from ACE2-A549 cells treated as in I. (K) TCID50 assay of conditioned medium from ACE2-A549 cells infected with SARS-CoV-2 0.1 MOI for 48 hours with or without pre-treatment of 0.1 μM diABZI-4. (L) Genome copy number analysis on ACE2-hESC-iAT2 cells infected with SARS-CoV-2 0.1 MOI for 72 hours with or without pre-treatment of 0.1 μM diABZI-4. (M) Immunofluorescence analysis of SARS-CoV-2 spike, Pro-SP-C and NKX2.1 in hESC-iAT2 cells infected with SARS-CoV-2 0.1 MOI for 72 hours with or without pre-treatment of 0.1 μM diABZI-4 A-B, M representative experiment. C-L pooled data from 3-5 independent experiments. *, P<0.05; ***, P<0.001; ****, P<0.0001 (E-K, two-way ANOVA). Error bars show means ± SEM.
” data-icon-position=”” data-hide-link-title=”0″>
diABZI-4 inhibits SARS-CoV-2 replication in lung epithelial cells. (A) Native immunoblot of STING dimerization from ACE-A549 cells treated with 0.1 μM of diABZI-4 for the indicated times. (B) Immunoblot analysis of p-IRF3, STING, ACE2 and β-actin from ACE2-A549 cells treated with 0.1 μM of diABZI-4 for the indicated times. (C-D) QPCR analysis of IFNβ (C) and TNF-α (D) mRNA expression in ACE2-A549 cells treated with 0.1 μM of diABZI-4 for 1.5 hours. (E) QPCR analysis of HCoV-OC43-N mRNA from ACE2-A549 cells infected with HCoV-OC43 0.1 MOI for 24 hours with or without pre-treatment of 0.1 μM diABZI-4. (F) TCID50 assay of conditioned medium from ACE2-A549 cells treated as in E for 48 hours. (G-I) QPCR analysis of SARS-CoV-2-N (G), Nsp14 (H) and ORF1 (I) mRNA from ACE2-A549 cells infected with SARS-CoV-2 0.1 MOI for 24 hours with or without pre-treatment of 0.1 μM diABZI-4. (J) Genome copy number analysis on conditioned medium from ACE2-A549 cells treated as in I. (K) TCID50 assay of conditioned medium from ACE2-A549 cells infected with SARS-CoV-2 0.1 MOI for 48 hours with or without pre-treatment of 0.1 μM diABZI-4. (L) Genome copy number analysis on ACE2-hESC-iAT2 cells infected with SARS-CoV-2 0.1 MOI for 72 hours with or without pre-treatment of 0.1 μM diABZI-4. (M) Immunofluorescence analysis of SARS-CoV-2 spike, Pro-SP-C and NKX2.1 in hESC-iAT2 cells infected with SARS-CoV-2 0.1 MOI for 72 hours with or without pre-treatment of 0.1 μM diABZI-4 A-B, M representative experiment. C-L pooled data from 3-5 independent experiments. *, P<0.05; ***, P<0.001; ****, P<0.0001 (E-K, two-way ANOVA). Error bars show means ± SEM.